Overview of the Study
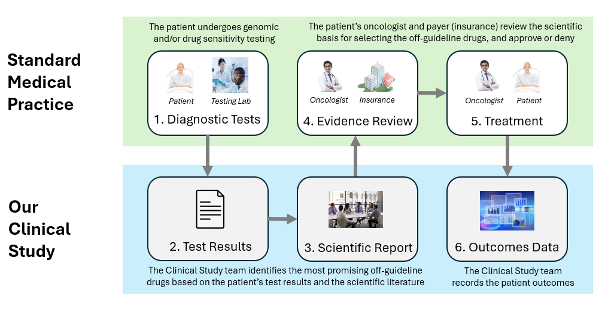
- Treatment Selection Test: The patient must undergo one or more treatment selection tests, which may include genomic tests or DSTs.
- Test Results: The Cancer Commons clinical study team assesses the treatment selection test results to identify any promising FDA-approved off-guideline drugs. If no promising drugs are identified, the process is stopped, and the patient is not included in the study.
- Scientific Evidence Report: If the test results indicate promising FDA-approved off-guideline drugs, the Cancer Commons clinical study team writes a Scientific Evidence Report. This report uses the test results and any supporting scientific literature to present the scientific evidence for using the off-guideline drugs to the patient’s oncologist and the payer of the drugs (Medicare, private insurance, or other). For a sample Scientific Evidence Report please visit www.cancercommons.org/sample_report
- Evidence Review: The oncologist and the insurance provider review the Scientific Evidence Report. If the oncologist consents to the treatment and the necessary arrangements for drug access through insurance reimbursement or other means are made, the patient is enrolled in the clinical study.
- Treatment: The patient's oncologist administers the recommended FDA-approved off-guideline drug or drugs. It is important to note that the oncologist independently makes all treatment decisions, and the clinical study team does not influence these decisions. The oncologist may choose not to proceed with treatment at any time, for any reason or no reason at all.
- Outcome Data: Following treatment, the clinical study team contacts the oncologist to gather a report on the patient's response to the off-guideline drugs. The treatment and outcome data are then entered into the study database to assess the predictive accuracy of the treatment selection tests.
How to Decide if the Study is Right for You
Here is our guidance on determining whether CCCS001 is suitable for you compared to opting for a clinical trial:
- If you are eligible for one or more high-value clinical trials that offer a significant potential benefit, it is advisable to prioritize those trials before considering CCCS001.
- If you are not eligible for or cannot access any clinical trials, then strongly consider CCCS001, subject to an analysis of the enrollment requirements described below.
- If you are eligible for a low-value clinical trial with limited potential for significant benefit, and also for CCCS001, consider that the average successful clinical trial results in an additional 2.1 months of overall survival for participants. This is on the lower end of the effects typically seen with FDA-approved drugs.
Determining if You Are Eligible for the Study
In addition to being over 18 years old and signing the Informed Consent Form, you must meet four inclusion criteria:
- You must have a valid genomic or functional test, or be willing to undergo a new test.
- The analysis of your test must yield a set of promising off-guideline drugs.
- Oncologist must be willing to administer one or more promising off-guideline drugs.
- There must be an approved method of paying for the administered off-guideline drug or drugs.
Treatment Selection Test
You must undergo a treatment selection test, which can be a genomic test, a DST, or both. Once you have the test results, submit them to the Cancer Commons Clinical Study Team through the Lead Clinical Research Nurse, Unsha Bakker (unsha.bakker@cancercommons.org). The Clinical Study Team will review the test results to determine your eligibility for the study. If you are deemed ineligible based on the test results, further testing might render you eligible.
Promising Off-Guideline Drugs
If your test results indicate one or more promising off-guideline drugs, the clinical study team will investigate the medical and scientific literature to find additional evidence supporting the use of these drugs for patients with similar cancers. If the combination of the test results and scientific support does not identify any promising off-guideline drugs, you will not be enrolled in the study.
Treating Oncologist
If the analysis of your test and the scientific and medical literature identifies one or more promising off-guideline drugs, the oncologist must review the evidence and declare their willingness to treat you with these drugs. Note that many oncologists face institutional constraints that prevent them from using off-guideline drugs under any circumstances. Additionally, the treating oncologist independently makes all treatment decisions and can decline to treat at any time, for any reason or no reason. If the oncologist is unable or unwilling to treat you with off-guideline drugs, you may be able to find another oncologist willing and able to do so. You may contact the study CRC, Unsha Bakker, for guidance on finding such oncologists.
Access to Off-Guideline Drugs
Unlike drugs in clinical trials, which are free to participants, FDA-approved off-guideline drugs are marketed and have established prices. Insurance companies are often reluctant to pay for off-guideline drugs but may do so under the doctrine of 'medical necessity.' The Cancer Commons study team will create the documentation typically required to obtain reimbursement for off-guideline drugs, which includes:
- A scientific report detailing the treatment selection test results and any supporting scientific literature, providing evidence that the off-guideline drug may be effective for you.
- A medical necessity cover letter, which you must sign, to be sent to the insurance company requesting reimbursement for the off-guideline drug or drugs based on medical necessity.
You will not be enrolled in the study until all the above inclusion criteria are met. If you believe you are eligible and they agree to enroll in this study, please contact Unsha Bakker, the Lead Clinical Research Nurse, at unsha.bakker@cancercommons.org.