This overview outlines the Cancer Commons observational clinical study CCCS001 in three parts
Part 1 explains the study's purpose and design, highlighting key components such as off-guideline drugs and treatment selection tests.
Part 2 describes the step-by-step process and flow of the study.
Part 3 describes the mechanics of the study: the inclusion/exclusion criteria and other elements of the study protocol.
Part 1
Purpose and Design of the Clinical Study
The aim of this clinical study is to demonstrate that treatment selection tests can identify effective FDA-approved drugs not currently accessible to patients under the NCCN Guidelines.
On-Guideline and Off-Guideline Drugs
“On-Guideline” drugs are those included in the NCCN Guidelines for each type of cancer. These drugs have been demonstrated through randomized controlled clinical trials to be sufficiently safe and effective for patients with a specific cancer, leading to their FDA approval for that particular cancer.
“Off-Guideline” drugs are FDA-approved medications not listed in the NCCN Guidelines for cancers other than those for which they were originally approved. This exclusion does not mean the drug is ineffective for other cancers; rather, it indicates the absence of randomized controlled clinical trials proving the drug's safety and efficacy for those cancers, which is required for FDA approval and inclusion in the NCCN Guidelines.
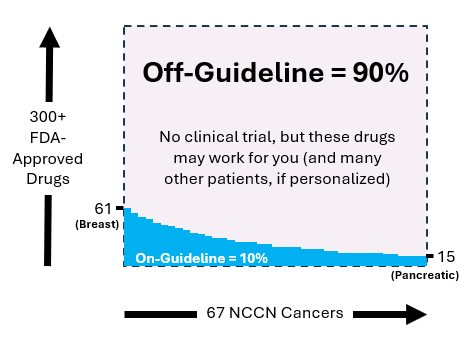
Figure 1 illustrates the distribution of on-guideline and off-guideline drugs. The horizontal axis represents the 67 cancers listed in the NCCN Guidelines, while the vertical axis shows over 300 FDA-approved cancer drugs. Breast cancer has the highest number of approved drugs at 61, whereas pancreatic cancer has the lowest at 15. The remaining cancers have between 15 and 61 FDA-approved on-guideline drugs.
Among the 67 NCCN cancers, 90% of all FDA-approved drugs are classified as off-guideline. This classification does not imply ineffectiveness for patients with those cancers. Instead, it indicates the absence of a clinical trial for that specific drug and cancer combination. Due to the high costs associated with clinical trials, many potential cancer-drug pairings remain untested.
Off-guideline drugs are generally not covered by insurance, whether Medicare or private, and as a result, oncologists typically do not prescribe them. These drugs, which make up 90% of FDA-approved but off-guideline cancer treatments, represent a vast potential for new therapies for advanced cancer patients who have exhausted other options.
Treatment Selection Tests
Treatment selection tests are diagnostic tests that can determine which treatments are most likely to be effective for a patient. There are two main types of treatment selection tests:
1. Genomic tests, which identify mutations that previous studies have determined are likely to be addressed by certain targeted therapies.
2. Drug sensitivity tests (DSTs), which directly measure the effects of a set of cancer drugs against a patient's live cancer cells (Figure 2).
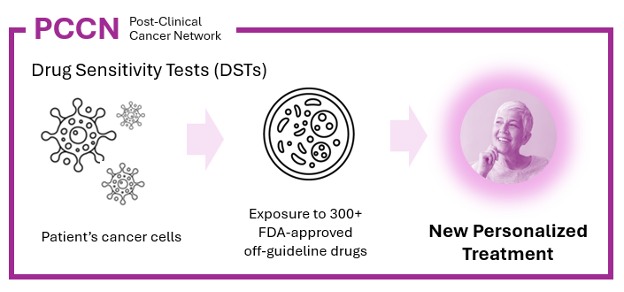
DSTs are incredibly powerful because they can be applied to any patient from whom enough live cancer cells can be collected, without needing prior studies to identify effective drugs. Unlike genomic tests, which require rare biomarkers, DSTs do not need any biomarkers and are applicable to nearly all cancer patients.
Scientific Evidence for Using Off-Guideline Drugs
The clinical study employs treatment selection tests, including both genomic tests and DSTs, to identify a promising set of off-guideline drugs for each patient. The Cancer Commons clinical study team, composed of PhD cancer experts, reviews the results of the patient's treatment selection tests. If the results are promising, they analyze the scientific literature for additional support for using one or more off-guideline drugs for the study participant. The test results and scientific literature are compiled into a Scientific Report, which is delivered to the patient's oncologist.
If the oncologist agrees that the identified off-guideline drugs are promising and is willing to treat the patient with the selected drug or drugs, the scientific report is then sent to the patient's health insurance provider (Medicare or private insurance). If the insurance provider approves reimbursement for the off-guideline drug or drugs, the oncologist proceeds to treat the patient.
In this scenario, the study's objective is fulfilled: patients who have exhausted effective treatment options are treated with off-guideline drugs. The patients' responses to these drugs, selected through treatment selection tests, are used to determine the predictive accuracy of the tests, which serves as the primary endpoint of this clinical study.
Part 2
Clinical Study Step-By-Step Process
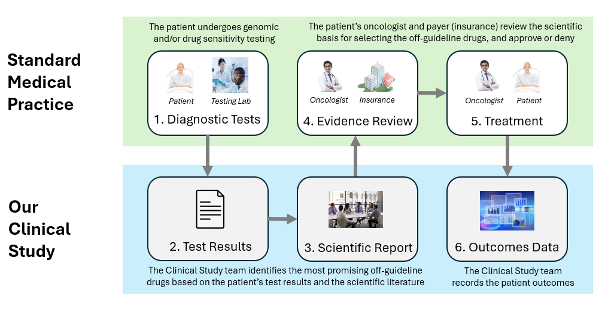
- Treatment Selection Test: The patient must undergo one or more treatment selection tests, which may include genomic tests or DSTs.
- Test Results: The Cancer Commons clinical study team assesses the treatment selection test results to identify any promising FDA-approved off-guideline drugs. If no promising drugs are identified, the process is stopped, and the patient is not included in the study.
- Scientific Evidence Report: If the test results indicate promising FDA-approved off-guideline drugs, the Cancer Commons clinical study team writes a Scientific Evidence Report. This report uses the test results and any supporting scientific literature to present the scientific evidence for using the off-guideline drugs to the patient’s oncologist and the payer of the drugs (Medicare, private insurance, or other). For a sample Scientific Evidence Report please visit www.cancercommons.org/sample_report
- Evidence Review: The oncologist and the insurance provider review the Scientific Evidence Report. If the oncologist consents to the treatment and the necessary arrangements for drug access through insurance reimbursement or other means are made, the patient is enrolled in the clinical study.
- Treatment: The patient's oncologist administers the recommended FDA-approved off-guideline drug or drugs. It is important to note that the oncologist independently makes all treatment decisions, and the clinical study team does not influence these decisions. The oncologist may choose not to proceed with treatment at any time, for any reason or no reason at all.
- Outcome Data: Following treatment, the clinical study team contacts the oncologist to gather a report on the patient's response to the off-guideline drugs. The treatment and outcome data are then entered into the study database to assess the predictive accuracy of the treatment selection tests.
Part 3
The Mechanics of the Study
In addition to being over 18 years old and signing the Informed Consent Form, the patient must meet four inclusion criteria:
- The patient must have a valid genomic or functional test or be willing to undergo a new test.
- The analysis of the patient's test must yield a set of promising off-guideline drugs.
- The patient’s oncologist must be willing to administer one or more promising off-guideline drugs.
- There must be an approved method of paying for the administered off-guideline drug or drugs.
The study initially includes three treatment selection tests:
1. Genomic mutation match
2. Genomic pathway match
3. Travera Drug SensitivityTest (DST)
In the future, we anticipate incorporating additional DSTs into the study, such as:
- Kyan/Mayo Test
- Pangea Enlight-NGS
- SEngine PARIS Test
- SageMedic Test
A key benefit of the study is its site-less nature. Since the treating oncologist is the patient's own oncologist, rather than one preselected for a specific study site, both the patient and their oncologist can participate in the study at any cancer hospital in the US.
A key drawback of the study is that a patient must meet three criteria: (1) they must have a treatment selection test that identifies promising FDA-approved off-guideline drugs, (2) their oncologist must be willing to administer such drugs, and (3) they must have access to the drugs, either through insurance or other means.
An unusual and valuable characteristic of the study is that each treatment selection test serves as an arm of the study. The first three arms are the genomic mutation match, the genomic pathway match, and the Travera RTS test. A patient who undergoes more than one of these tests will be entered into multiple arms of the study, allowing them to contribute predictive accuracy data for multiple treatment selection tests.