When facing a frightening new cancer diagnosis, some people ask their doctors, “What would you do if you were me?” Here, our Curious Dr. George asks leukemia expert Mark R. Litzow, MD, how he would handle his own hypothetical cancer case. Dr. Litzow is Professor Emeritus in the Division of Hematology at the Mayo Clinic in Minnesota.
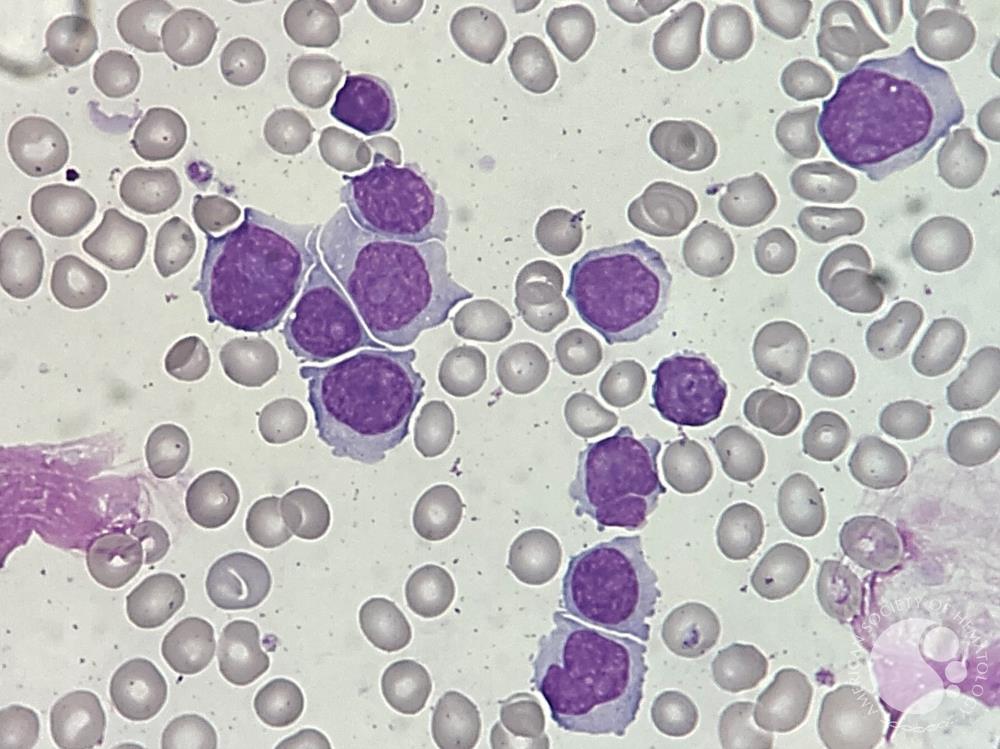
Curious Dr. George: Please consider this hypothetical scenario:You are an older white American male who has enjoyed good general health most of your life. Recently, while starting retirement, you have lost your appetite, developed a low-grade fever, and felt quite fatigued. And, for a few weeks you have accumulated more bruises than expected. Your primary care physician comments that you seem pale, and on examination, your spleen is palpable. A complete blood count (CBC) reveals pancytopenia with an elevated white blood cell count, and circulating blasts are visible on your blood smear. How would you proceed?
Mark R. Litzow, MD: To start, in real life, I am a 71-year-old retired hematologist. I had an NSTEMI (a type of heart attack) 2 years ago, with a 95% occlusion of my ramus intermedius artery that was successfully stented with a drug-eluting stent. I completed a year of dual anti-platelet therapy and now remain on low dose aspirin along with lisinopril and atorvastatin. Today, I remain symptom-free and am physically active, having completed a half-marathon in late April 2025. This would be my only significant co-morbidity.
Now, building on the hypothetical new onset of the symptoms you describe, let’s say the CBC shows a leukocyte count of 50×109/L with 45% circulating blasts, platelets of 20×109/L and hemoglobin of 8.0 g/dL. I then contact one of my trusted colleagues in the Mayo Clinic’s Acute Leukemia Group, who is able to see me in the outpatient clinic that same day. The CBC results are very concerning in light of a possible diagnosis of acute leukemia, and I am scared, to be honest. Having cared for acute leukemia patients my entire career, I have often asked myself what I would do if I was diagnosed with acute leukemia. I have always thought that if I remained in reasonably good health, I would likely want aggressive treatment, and would even consider a blood or marrow transplant, if that was warranted and associated with a reasonable chance of success.
To continue this scenario, on further evaluation, flow cytometric assessment of the peripheral blood shows the following immunophenotype of the blasts: CD45 weak, CD19+, CD10+, CD22+, CD24+, CD20-, CD34+, HLA-DR+, CD25+, CRLF2-, CD65/CD15-, CD13+. A bone marrow biopsy shows a hypercellular marrow at 90% cellularity with 75% blasts. Cytogenetic analysis shows the Philadelphia chromosome, t(9;22)(q34;q11) (Ph+) in all metaphases as a sole abnormality. Polymerase chain reaction testing is positive for a BCR:ABL1 gene abnormality expressing the p190 isoform. By fluorescent in situ hybridization (FISH) testing, an IKZF1 deletion is detected as a sole additional abnormality.
Stepping back to reality, the treatment of Ph+ acute lymphocytic leukemia (ALL) has evolved considerably in a favorable direction in recent years. The main advancement has been the addition of tyrosine kinase inhibitors (TKIs)—which were originally developed for the treatment of Ph+ chronic myeloid leukemia (CML)—to the treatment of Ph+ ALL. The leukemia group at MD Anderson Cancer Center has pioneered the addition of TKIs to their hyperCVAD combination chemotherapy regimen, and with the addition of the third generation TKI, ponatinib, has achieved a 6-year event free survival (EFS) rate of 65% and an overall survival (OS) rate of 75%. More recently, a TKI has been combined with an immunotherapeutic agent, the bispecific T-cell engaging (BiTE) blinatumomab, resulting in a 3-year EFS of 91% and OS 77%. Now, a phase 3, multicenter, U.S.-based clinical trial is randomizing patients between the ages of 18 and 70 to hyperCVAD plus ponatinib or another TKI, dasatinib (investigator’s choice), or to a combination of blinatumomab and ponatinib or dasatinib.
For myself, I would opt for enrollment in a clinical trial if one was available. With my age, I would not be eligible for the phase 3 multicenter trial. Outside a clinical trial I would opt for treatment with ponatinib and blinatumomab. Ponatinib is associated with an increased risk of cardiovascular (CV) complications, but is currently used in lower doses with further dose reductions based on treatment response, which has lessened the risk of CV complications. Given my stable cardiac status and symptom-free state, I would favor treatment with ponatinib.
Shortly after my diagnosis I would be hospitalized and started on prednisone at 60 mg/m2,which could continue for as long as 21 days, but as short as 7 days depending on how quickly my white blood cell count and blast count subside. I would simultaneously initiate therapy with ponatinib at 30 mg orally daily, and then add blinatumomab by continuous IV (CIV) infusion after the 7–21-day pre-phase, starting at a flat dose of 9 mcg per day with escalation to 28 mcg per day after 5-7 days if I remain stable. The MD Anderson regimen does not include a steroid pre-phase, but starts therapy immediately with ponatinib and blinatumomab. With this approach, they have seen a low risk—only 12%—of cytokine release syndrome with blinatumomab, with all cases being grade 1-2 in severity, so this would be a reasonable alternate approach. Importantly, I would need to receive frequent doses of intrathecal (IT) chemotherapy to help prevent a central nervous system relapse. Up to 15 doses of IT chemotherapy is recommended, given as 3 doses with each cycle of blinatumomab. Blinatumomab is given for 4 weeks by CIV, with a 2-week break between cycles. Depending on my response to therapy, up to 4-5 cycles of blinatumomab could be given.
I would want my response to therapy to be assessed with sensitive tests for measurable residual disease (MRD) and include testing by next-generation sequencing (NGS) with the clonoSEQ® assay, which assesses for immunoglobulin gene rearrangements up to a level of 10-6. I would also assess the level of the BCR-ABL1 gene by PCR, though studies have shown that as long as the NGS assay by clonoSEQ® is negative (which defines a complete molecular response [CMR]), positivity for the PCR test does not have prognostic significance and may represent mature cells with the BCR-ABL1 gene that are not able to replicate. Once I achieve a CMR, the ponatinib dose could be reduced to 15 mg daily and would then be continued indefinitely to reduce the risk of relapse.
Retrospective studies have shown that if a CMR is achieved within 3 months of diagnosis, there is no added benefit to pursuing an allogeneic hematopoietic cell transplant (alloHCT). In the past alloHCT was indicated for all patients with Ph+ ALL who were felt to be candidates, but now it is being applied less and less, given the efficacy of combining a TKI with blinatumomab or chemotherapy.
My initial disease features at diagnosis suggest that I have a reasonably good prognosis. Hopefully I would reach a CMR within 3 months and not require an alloHCT. Although it is generally recommended that ponatinib continue indefinitely as maintenance therapy, there are anecdotal reports of patients who stopped ponatinib maintenance therapy for varying reasons and did not relapse. So, similar to what has been reported with CML, it may be possible to achieve a treatment-free remission in Ph+ ALL as well.
Obviously, the diagnosis and treatment of Ph+ ALL is a major disrupter of one’s life, and I would seek out the support of family and friends to help guide me through the ups and downs of therapy and monitoring. I would attempt to maintain a regular exercise schedule and emphasize good nutrition throughout my treatment. I have a living will and an advance directive, and I would make sure these are up to date to reflect my current wishes. I would also focus on my spiritual life to help keep a broader perspective on the significance of my illness in the grand scheme of life. As I have observed with so many of my patients, I would attempt to maintain a good attitude and keep a sense of humor in the setting of this life-threatening illness.
Dr. Litzow can be reached at litzow.mark@mayo.edu.